HYMPAVZI™ Full Patient Information
(marstacimab-hncq)
Full Patient Information
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Ensure that patients and caregivers who will administer HYMPAVZI receive appropriate training and instruction on the proper storage, use and handling of HYMPAVZI from a healthcare professional.
Thromboembolic Events
Inform patients and/or caregivers that HYMPAVZI increases coagulation potential. Discuss the appropriate dosing of concomitant agents such as FVIII or FIX with the patient prior to starting on HYMPAVZI prophylaxis [see Warnings and Precautions (5.1)]. Advise the patient to seek immediate medical attention if any signs or symptoms of thromboembolism occur.
Hypersensitivity
Inform patients and/or caregivers that hypersensitivity reactions such as rash and pruritus are possible. Advise patients to discontinue HYMPAVZI and seek immediate emergency treatment if a severe hypersensitivity reaction occurs [see Warnings and Precautions (5.2)].
Pregnancy
Advise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose. Advise patients to report known pregnancies [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about HYMPAVZI, please visit www.pfizermedinfo.com or call 1-800-438-1985.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1556-1.0
Patient Package Insert
| This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 | ||
PATIENT INFORMATION HYMPAVZI (him-PAV-zee) (marstacimab-hncq) injection, for subcutaneous use | ||
Important information: Before you start using HYMPAVZI, it is very important to talk to your healthcare provider about using factor VIII and factor IX products (products that help blood clot but work in a different way than HYMPAVZI). You may need to use factor VIII or factor IX medicines to treat episodes of breakthrough bleeding during treatment with HYMPAVZI. Carefully follow your healthcare provider’s instructions regarding when to use factor VIII or factor IX medicines and the prescribed dose during your treatment with HYMPAVZI. | ||
What is HYMPAVZI? HYMPAVZI is a prescription medicine used to prevent or reduce the frequency of bleeding episodes in adults and children 12 years of age and older with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors. It is not known if HYMPAVZI is safe and effective in children younger than 12 years old. | ||
Before using HYMPAVZI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription medicines, over‑the‑counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | ||
How should I use HYMPAVZI? See the detailed “Instructions for Use” that comes with your HYMPAVZI for information on how to inject a dose of HYMPAVZI, and how to properly throw away (dispose of) used HYMPAVZI prefilled syringe or HYMPAVZI prefilled pen.
| ||
What are the possible side effects of HYMPAVZI? HYMPAVZI may cause serious side effects, including:
| ||
|
| |
| ||
|
| |
The most common side effects of HYMPAVZI are injection site reactions, including: | ||
|
| |
These are not all of the possible side effects of HYMPAVZI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store HYMPAVZI?
Keep HYMPAVZI and all medicines out of the reach of children. | ||
General information about the safe and effective use of HYMPAVZI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYMPAVZI for a condition for which it was not prescribed. Do not give HYMPAVZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYMPAVZI that is written for health professionals. | ||
What are the ingredients in HYMPAVZI? Active ingredient: marstacimab‑hncq Inactive ingredients: edetate disodium, histidine, L-histidine monohydrochloride, polysorbate 80, sucrose, and water for injection | ||
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
| ||
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Syringe and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Syringe is a Single-Dose Prefilled Syringe (called “Syringe” in this Instructions for Use). The HYMPAVZI Prefilled Syringe contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (Exp) printed on the HYMPAVZI Prefilled Syringe.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
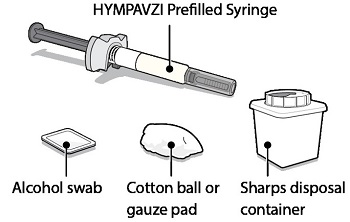
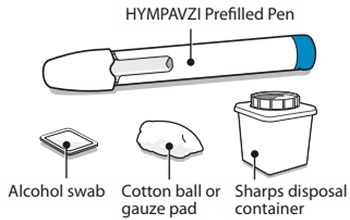
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Syringe
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Syringe disposal (see “Step 11 – Disposal of Syringe” and “Safe Syringe Disposal” information section)
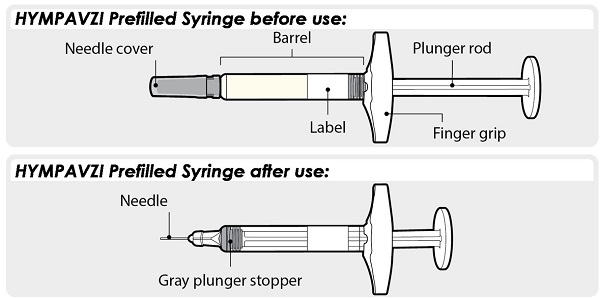
HYMPAVZI Prefilled Syringe
Always hold HYMPAVZI Prefilled Syringe by the barrel to prevent damage.
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Syringe from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Syringe label.
- •
- Check the Syringe for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the needle cover until you are ready to inject.
- •
- Throw away (dispose of) the Syringe if it is damaged, or if the Syringe or the carton containing the Syringe has been dropped.
- •
- Do not use the Syringe if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Syringe to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Syringe, such as warming the Syringe in a microwave or hot water.
Step 2 – Check Expiration Date
- •
- Check the expiration date (Exp) printed on the Syringe label.
- •
- Do not use if the expiration date (Exp) has passed.
Step 3 – Check Medicine
- •
- Gently tilt the Syringe back and forth.
- •
- Look carefully at the medicine in the Syringe.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Syringe if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the Syringe.
If you have any questions about the medicine, contact your healthcare provider.
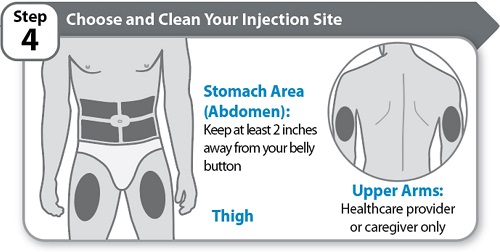
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your upper arms by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
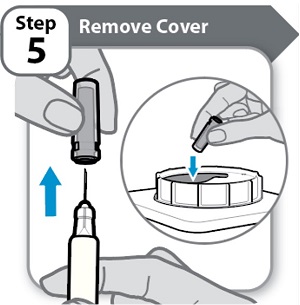
Step 5 – Remove Cover
- •
- Hold the Syringe by the barrel.
- •
- Pull the needle cover straight off carefully.
- •
- Put the needle cover into an FDA-cleared sharps disposal container right away. You will not need it again.
- •
- Do not touch the needle or let it touch any surfaces.
Note: It is normal to see a few drops of medicine at the needle tip.
Caution: Handle the Syringe with care to avoid an accidental needle injury.
Injecting HYMPAVZI
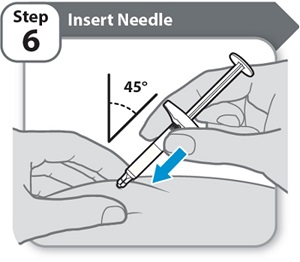
Step 6 – Insert Needle
- •
- Pinch your cleaned skin between your thumb and fingers to create a firm surface.
- •
- Fully insert the needle into your skin at a 45° angle, as shown. Do not hold or push on the plunger while inserting the needle.
Keep your skin pinched throughout the injection.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Syringe and get a new HYMPAVZI Prefilled Syringe.
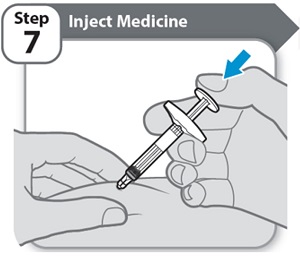
Step 7 – Inject Medicine
- •
- Slowly inject all of HYMPAVZI by gently pushing the plunger rod all the way down, until the barrel is empty.
Note: Count slowly to 5 after the plunger rod has been fully pushed down before removing the needle from your skin.
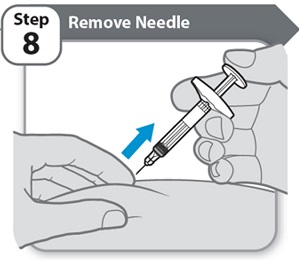
Step 8 – Remove Needle
- •
- Pull the needle and Syringe out of your skin at the same angle as inserted.
Note: If you see a small drop of medicine on your skin, wait a little longer before removing the needle when you give your next injection.
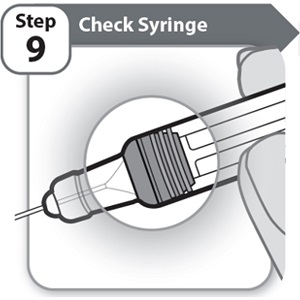
Step 9 – Check Syringe
- •
- Check the Syringe to make sure the gray plunger stopper is in the position shown.
If the gray plunger stopper is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Never re-insert the needle.
Do not inject another dose.
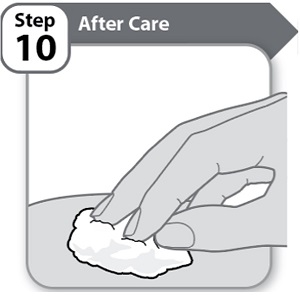
Step 10 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-10. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
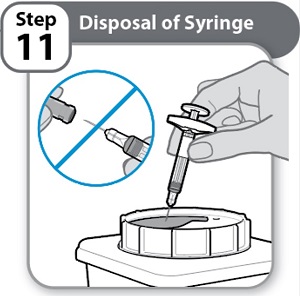
- •
- Put the used Syringe in an FDA-cleared sharps disposal container right away after use.
Never re-cap the needle.
- •
- Do not throw away (dispose of) Syringes in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Syringe Disposal” information section.
- •
- Always throw away (dispose of) Syringes in a sharps disposal container. Do not dispose of Syringes in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1576-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled pen
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Pen and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Pen is a Single-Dose Prefilled Pen (called “Pen” in this Instructions for Use). The HYMPAVZI Prefilled Pen contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (EXP) printed on the HYMPAVZI Prefilled Pen.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Pen
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Pen disposal (see “Step 10 – Disposal of Pen” and “Safe Pen Disposal” information section)
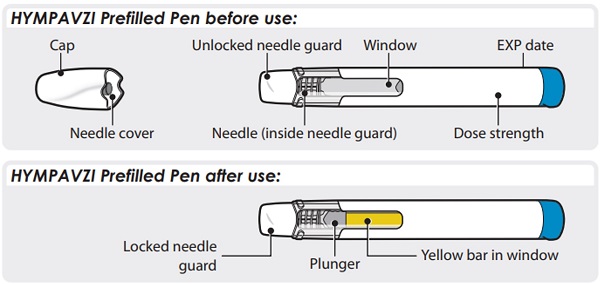
HYMPAVZI Prefilled Pen
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Pen from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Pen label.
- •
- Check the Pen for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the cap until you are ready to inject.
- •
- Throw away (dispose of) the Pen if it is damaged, or if the Pen or the carton containing the Pen has been dropped.
- •
- Do not use the Pen if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Pen to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Pen, such as warming the Pen in a microwave or hot water.
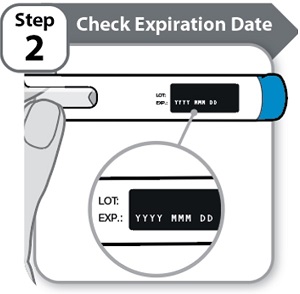
Step 2 – Check Expiration Date
- •
- Check the expiration date (EXP) printed on the Pen label.
- •
- Do not use if the expiration date (EXP) has passed.
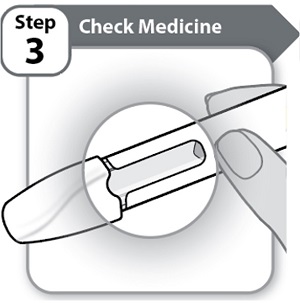
Step 3 – Check Medicine
- •
- Look carefully at the medicine through the window on the Pen.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Pen if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the window.
If you have any questions about the medicine, contact your healthcare provider.
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your buttocks by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
Step 5 – Twist Off Cap
- •
- Twist and pull off the cap.
- •
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
Note:
- o
- It is normal to see a few drops of medicine at the needle tip.
- o
- The needle cover will stay inside the cap after cap removal.
Caution: Handle the Pen with care as it contains a needle.
Do not put or press your hand over the needle guard. Doing so may cause a needle injury.
Injecting HYMPAVZI
Step 6 – Inject Medicine
- •
- Hold the Pen straight (at 90° angle) against your cleaned skin so you can see the window.
- •
- Push the Pen down firmly straight against your skin and keep pushing until the injection is complete. You will hear the 1st click when the injection starts.
- •
- Keep pushing the Pen firmly against your skin while the yellow bar moves across the window. You will hear a 2nd click when the injection is almost complete.
- •
- Count slowly to 5 after you hear the 2nd click to make sure you get a full dose.
Do not remove the Pen from your skin until you have counted slowly to 5 after you hear the 2nd click and until the yellow marker completely fills the window (see “Step 7 – Remove Pen”).
Note: The needle goes into your skin as you push the Pen down. Your healthcare provider may suggest gently pinching your skin while you inject.
Note: If you do not hear a click when pushing the Pen against your skin, try pushing down harder. If you still cannot start the injection, get a new HYMPAVZI Prefilled Pen.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Pen and get a new HYMPAVZI Prefilled Pen.
- •
- Remove the Pen from your skin.
- o
- If you see a small drop of medicine on your skin, wait a little longer before removing the Pen when you give your next injection.
Note: After you remove the Pen from your skin, the needle guard will automatically cover the needle and lock in place.
The Pen cannot be reused.
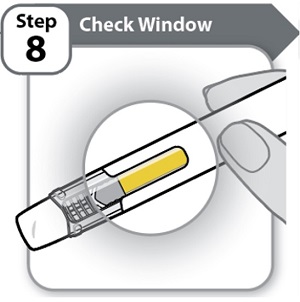
Step 8 – Check Window
- •
- Check the window to make sure all the medicine has been injected.
If the yellow bar is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Do not inject another dose.
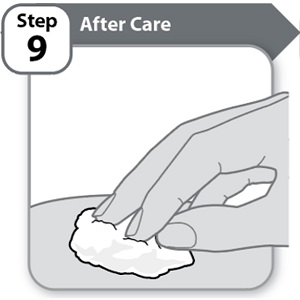
Step 9 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-9. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
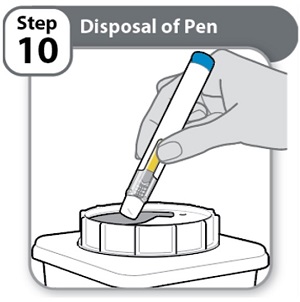
- •
- Put the used Pen in an FDA-cleared sharps disposal container right away after use.
- •
- Do not throw away (dispose of) Pens in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Pen Disposal” information section.
- •
- Always throw away (dispose of) Pens in a sharps disposal container. Do not dispose of Pens in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1577-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Find HYMPAVZI™ medical information:

Find HYMPAVZI™ medical information:
HYMPAVZI™ Quick Finder
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Ensure that patients and caregivers who will administer HYMPAVZI receive appropriate training and instruction on the proper storage, use and handling of HYMPAVZI from a healthcare professional.
Thromboembolic Events
Inform patients and/or caregivers that HYMPAVZI increases coagulation potential. Discuss the appropriate dosing of concomitant agents such as FVIII or FIX with the patient prior to starting on HYMPAVZI prophylaxis [see Warnings and Precautions (5.1)]. Advise the patient to seek immediate medical attention if any signs or symptoms of thromboembolism occur.
Hypersensitivity
Inform patients and/or caregivers that hypersensitivity reactions such as rash and pruritus are possible. Advise patients to discontinue HYMPAVZI and seek immediate emergency treatment if a severe hypersensitivity reaction occurs [see Warnings and Precautions (5.2)].
Pregnancy
Advise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose. Advise patients to report known pregnancies [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about HYMPAVZI, please visit www.pfizermedinfo.com or call 1-800-438-1985.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1556-1.0
Patient Package Insert
| This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 | ||
PATIENT INFORMATION HYMPAVZI (him-PAV-zee) (marstacimab-hncq) injection, for subcutaneous use | ||
Important information: Before you start using HYMPAVZI, it is very important to talk to your healthcare provider about using factor VIII and factor IX products (products that help blood clot but work in a different way than HYMPAVZI). You may need to use factor VIII or factor IX medicines to treat episodes of breakthrough bleeding during treatment with HYMPAVZI. Carefully follow your healthcare provider’s instructions regarding when to use factor VIII or factor IX medicines and the prescribed dose during your treatment with HYMPAVZI. | ||
What is HYMPAVZI? HYMPAVZI is a prescription medicine used to prevent or reduce the frequency of bleeding episodes in adults and children 12 years of age and older with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors. It is not known if HYMPAVZI is safe and effective in children younger than 12 years old. | ||
Before using HYMPAVZI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription medicines, over‑the‑counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | ||
How should I use HYMPAVZI? See the detailed “Instructions for Use” that comes with your HYMPAVZI for information on how to inject a dose of HYMPAVZI, and how to properly throw away (dispose of) used HYMPAVZI prefilled syringe or HYMPAVZI prefilled pen.
| ||
What are the possible side effects of HYMPAVZI? HYMPAVZI may cause serious side effects, including:
| ||
|
| |
| ||
|
| |
The most common side effects of HYMPAVZI are injection site reactions, including: | ||
|
| |
These are not all of the possible side effects of HYMPAVZI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store HYMPAVZI?
Keep HYMPAVZI and all medicines out of the reach of children. | ||
General information about the safe and effective use of HYMPAVZI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYMPAVZI for a condition for which it was not prescribed. Do not give HYMPAVZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYMPAVZI that is written for health professionals. | ||
What are the ingredients in HYMPAVZI? Active ingredient: marstacimab‑hncq Inactive ingredients: edetate disodium, histidine, L-histidine monohydrochloride, polysorbate 80, sucrose, and water for injection | ||
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
| ||
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Syringe and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Syringe is a Single-Dose Prefilled Syringe (called “Syringe” in this Instructions for Use). The HYMPAVZI Prefilled Syringe contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (Exp) printed on the HYMPAVZI Prefilled Syringe.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Syringe
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Syringe disposal (see “Step 11 – Disposal of Syringe” and “Safe Syringe Disposal” information section)
HYMPAVZI Prefilled Syringe
Always hold HYMPAVZI Prefilled Syringe by the barrel to prevent damage.
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Syringe from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Syringe label.
- •
- Check the Syringe for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the needle cover until you are ready to inject.
- •
- Throw away (dispose of) the Syringe if it is damaged, or if the Syringe or the carton containing the Syringe has been dropped.
- •
- Do not use the Syringe if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Syringe to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Syringe, such as warming the Syringe in a microwave or hot water.
Step 2 – Check Expiration Date
- •
- Check the expiration date (Exp) printed on the Syringe label.
- •
- Do not use if the expiration date (Exp) has passed.
Step 3 – Check Medicine
- •
- Gently tilt the Syringe back and forth.
- •
- Look carefully at the medicine in the Syringe.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Syringe if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the Syringe.
If you have any questions about the medicine, contact your healthcare provider.
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your upper arms by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
Step 5 – Remove Cover
- •
- Hold the Syringe by the barrel.
- •
- Pull the needle cover straight off carefully.
- •
- Put the needle cover into an FDA-cleared sharps disposal container right away. You will not need it again.
- •
- Do not touch the needle or let it touch any surfaces.
Note: It is normal to see a few drops of medicine at the needle tip.
Caution: Handle the Syringe with care to avoid an accidental needle injury.
Injecting HYMPAVZI
Step 6 – Insert Needle
- •
- Pinch your cleaned skin between your thumb and fingers to create a firm surface.
- •
- Fully insert the needle into your skin at a 45° angle, as shown. Do not hold or push on the plunger while inserting the needle.
Keep your skin pinched throughout the injection.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Syringe and get a new HYMPAVZI Prefilled Syringe.
Step 7 – Inject Medicine
- •
- Slowly inject all of HYMPAVZI by gently pushing the plunger rod all the way down, until the barrel is empty.
Note: Count slowly to 5 after the plunger rod has been fully pushed down before removing the needle from your skin.
Step 8 – Remove Needle
- •
- Pull the needle and Syringe out of your skin at the same angle as inserted.
Note: If you see a small drop of medicine on your skin, wait a little longer before removing the needle when you give your next injection.
Step 9 – Check Syringe
- •
- Check the Syringe to make sure the gray plunger stopper is in the position shown.
If the gray plunger stopper is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Never re-insert the needle.
Do not inject another dose.
Step 10 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-10. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Put the used Syringe in an FDA-cleared sharps disposal container right away after use.
Never re-cap the needle.
- •
- Do not throw away (dispose of) Syringes in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Syringe Disposal” information section.
- •
- Always throw away (dispose of) Syringes in a sharps disposal container. Do not dispose of Syringes in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1576-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled pen
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Pen and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Pen is a Single-Dose Prefilled Pen (called “Pen” in this Instructions for Use). The HYMPAVZI Prefilled Pen contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (EXP) printed on the HYMPAVZI Prefilled Pen.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Pen
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Pen disposal (see “Step 10 – Disposal of Pen” and “Safe Pen Disposal” information section)
HYMPAVZI Prefilled Pen
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Pen from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Pen label.
- •
- Check the Pen for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the cap until you are ready to inject.
- •
- Throw away (dispose of) the Pen if it is damaged, or if the Pen or the carton containing the Pen has been dropped.
- •
- Do not use the Pen if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Pen to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Pen, such as warming the Pen in a microwave or hot water.
Step 2 – Check Expiration Date
- •
- Check the expiration date (EXP) printed on the Pen label.
- •
- Do not use if the expiration date (EXP) has passed.
Step 3 – Check Medicine
- •
- Look carefully at the medicine through the window on the Pen.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Pen if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the window.
If you have any questions about the medicine, contact your healthcare provider.
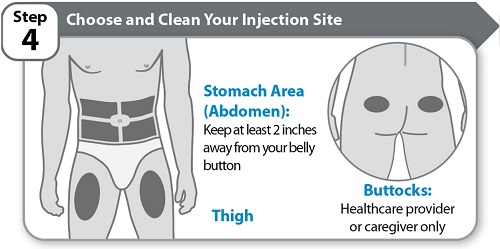
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your buttocks by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
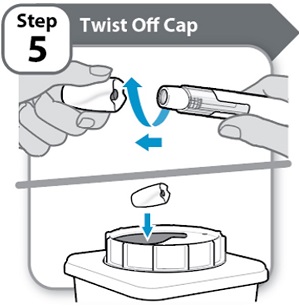
Step 5 – Twist Off Cap
- •
- Twist and pull off the cap.
- •
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
Note:
- o
- It is normal to see a few drops of medicine at the needle tip.
- o
- The needle cover will stay inside the cap after cap removal.
Caution: Handle the Pen with care as it contains a needle.
Do not put or press your hand over the needle guard. Doing so may cause a needle injury.
Injecting HYMPAVZI
Step 6 – Inject Medicine
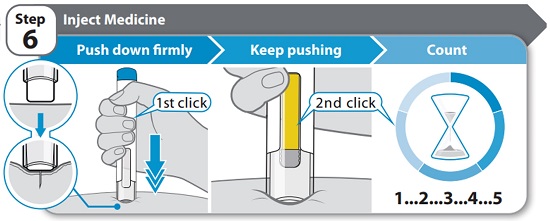
- •
- Hold the Pen straight (at 90° angle) against your cleaned skin so you can see the window.
- •
- Push the Pen down firmly straight against your skin and keep pushing until the injection is complete. You will hear the 1st click when the injection starts.
- •
- Keep pushing the Pen firmly against your skin while the yellow bar moves across the window. You will hear a 2nd click when the injection is almost complete.
- •
- Count slowly to 5 after you hear the 2nd click to make sure you get a full dose.
Do not remove the Pen from your skin until you have counted slowly to 5 after you hear the 2nd click and until the yellow marker completely fills the window (see “Step 7 – Remove Pen”).
Note: The needle goes into your skin as you push the Pen down. Your healthcare provider may suggest gently pinching your skin while you inject.
Note: If you do not hear a click when pushing the Pen against your skin, try pushing down harder. If you still cannot start the injection, get a new HYMPAVZI Prefilled Pen.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Pen and get a new HYMPAVZI Prefilled Pen.
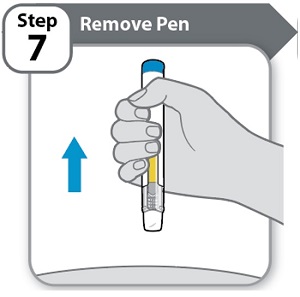
- •
- Remove the Pen from your skin.
- o
- If you see a small drop of medicine on your skin, wait a little longer before removing the Pen when you give your next injection.
Note: After you remove the Pen from your skin, the needle guard will automatically cover the needle and lock in place.
The Pen cannot be reused.
Step 8 – Check Window
- •
- Check the window to make sure all the medicine has been injected.
If the yellow bar is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Do not inject another dose.
Step 9 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-9. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Put the used Pen in an FDA-cleared sharps disposal container right away after use.
- •
- Do not throw away (dispose of) Pens in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Pen Disposal” information section.
- •
- Always throw away (dispose of) Pens in a sharps disposal container. Do not dispose of Pens in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1577-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Health Professional Information
Full Patient Information
Full Patient Information
17 PATIENT COUNSELING INFORMATION
- •
- Advise the patient and/or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Ensure that patients and caregivers who will administer HYMPAVZI receive appropriate training and instruction on the proper storage, use and handling of HYMPAVZI from a healthcare professional.
Thromboembolic Events
Inform patients and/or caregivers that HYMPAVZI increases coagulation potential. Discuss the appropriate dosing of concomitant agents such as FVIII or FIX with the patient prior to starting on HYMPAVZI prophylaxis [see Warnings and Precautions (5.1)]. Advise the patient to seek immediate medical attention if any signs or symptoms of thromboembolism occur.
Hypersensitivity
Inform patients and/or caregivers that hypersensitivity reactions such as rash and pruritus are possible. Advise patients to discontinue HYMPAVZI and seek immediate emergency treatment if a severe hypersensitivity reaction occurs [see Warnings and Precautions (5.2)].
Pregnancy
Advise female patients of reproductive potential to use effective contraception during treatment with HYMPAVZI and for 2 months after the last dose. Advise patients to report known pregnancies [see Warnings and Precautions (5.3) and Use in Specific Populations (8.1, 8.3)].
This product’s labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
For medical information about HYMPAVZI, please visit www.pfizermedinfo.com or call 1-800-438-1985.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1556-1.0
Patient Package Insert
| This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 10/2024 | ||
PATIENT INFORMATION HYMPAVZI (him-PAV-zee) (marstacimab-hncq) injection, for subcutaneous use | ||
Important information: Before you start using HYMPAVZI, it is very important to talk to your healthcare provider about using factor VIII and factor IX products (products that help blood clot but work in a different way than HYMPAVZI). You may need to use factor VIII or factor IX medicines to treat episodes of breakthrough bleeding during treatment with HYMPAVZI. Carefully follow your healthcare provider’s instructions regarding when to use factor VIII or factor IX medicines and the prescribed dose during your treatment with HYMPAVZI. | ||
What is HYMPAVZI? HYMPAVZI is a prescription medicine used to prevent or reduce the frequency of bleeding episodes in adults and children 12 years of age and older with hemophilia A without factor VIII inhibitors or hemophilia B without factor IX inhibitors. It is not known if HYMPAVZI is safe and effective in children younger than 12 years old. | ||
Before using HYMPAVZI, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription medicines, over‑the‑counter medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. | ||
How should I use HYMPAVZI? See the detailed “Instructions for Use” that comes with your HYMPAVZI for information on how to inject a dose of HYMPAVZI, and how to properly throw away (dispose of) used HYMPAVZI prefilled syringe or HYMPAVZI prefilled pen.
| ||
What are the possible side effects of HYMPAVZI? HYMPAVZI may cause serious side effects, including:
| ||
|
| |
| ||
|
| |
The most common side effects of HYMPAVZI are injection site reactions, including: | ||
|
| |
These are not all of the possible side effects of HYMPAVZI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | ||
How should I store HYMPAVZI?
Keep HYMPAVZI and all medicines out of the reach of children. | ||
General information about the safe and effective use of HYMPAVZI. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use HYMPAVZI for a condition for which it was not prescribed. Do not give HYMPAVZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about HYMPAVZI that is written for health professionals. | ||
What are the ingredients in HYMPAVZI? Active ingredient: marstacimab‑hncq Inactive ingredients: edetate disodium, histidine, L-histidine monohydrochloride, polysorbate 80, sucrose, and water for injection | ||
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
| ||
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled syringe
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Syringe and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Syringe is a Single-Dose Prefilled Syringe (called “Syringe” in this Instructions for Use). The HYMPAVZI Prefilled Syringe contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (Exp) printed on the HYMPAVZI Prefilled Syringe.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Syringe
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Syringe disposal (see “Step 11 – Disposal of Syringe” and “Safe Syringe Disposal” information section)
HYMPAVZI Prefilled Syringe
Always hold HYMPAVZI Prefilled Syringe by the barrel to prevent damage.
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Syringe from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Syringe label.
- •
- Check the Syringe for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the needle cover until you are ready to inject.
- •
- Throw away (dispose of) the Syringe if it is damaged, or if the Syringe or the carton containing the Syringe has been dropped.
- •
- Do not use the Syringe if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Syringe to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Syringe, such as warming the Syringe in a microwave or hot water.
Step 2 – Check Expiration Date
- •
- Check the expiration date (Exp) printed on the Syringe label.
- •
- Do not use if the expiration date (Exp) has passed.
Step 3 – Check Medicine
- •
- Gently tilt the Syringe back and forth.
- •
- Look carefully at the medicine in the Syringe.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Syringe if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the Syringe.
If you have any questions about the medicine, contact your healthcare provider.
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your upper arms by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
Step 5 – Remove Cover
- •
- Hold the Syringe by the barrel.
- •
- Pull the needle cover straight off carefully.
- •
- Put the needle cover into an FDA-cleared sharps disposal container right away. You will not need it again.
- •
- Do not touch the needle or let it touch any surfaces.
Note: It is normal to see a few drops of medicine at the needle tip.
Caution: Handle the Syringe with care to avoid an accidental needle injury.
Injecting HYMPAVZI
Step 6 – Insert Needle
- •
- Pinch your cleaned skin between your thumb and fingers to create a firm surface.
- •
- Fully insert the needle into your skin at a 45° angle, as shown. Do not hold or push on the plunger while inserting the needle.
Keep your skin pinched throughout the injection.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Syringe and get a new HYMPAVZI Prefilled Syringe.
Step 7 – Inject Medicine
- •
- Slowly inject all of HYMPAVZI by gently pushing the plunger rod all the way down, until the barrel is empty.
Note: Count slowly to 5 after the plunger rod has been fully pushed down before removing the needle from your skin.
Step 8 – Remove Needle
- •
- Pull the needle and Syringe out of your skin at the same angle as inserted.
Note: If you see a small drop of medicine on your skin, wait a little longer before removing the needle when you give your next injection.
Step 9 – Check Syringe
- •
- Check the Syringe to make sure the gray plunger stopper is in the position shown.
If the gray plunger stopper is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Never re-insert the needle.
Do not inject another dose.
Step 10 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-10. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Put the used Syringe in an FDA-cleared sharps disposal container right away after use.
Never re-cap the needle.
- •
- Do not throw away (dispose of) Syringes in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Syringe Disposal” information section.
- •
- Always throw away (dispose of) Syringes in a sharps disposal container. Do not dispose of Syringes in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1576-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Instructions for Use
INSTRUCTIONS FOR USE
HYMPAVZI™ (him-PAV-zee)
(marstacimab-hncq)
injection, for subcutaneous use
single-dose prefilled pen
This Instructions for Use contains information on how to inject HYMPAVZI.
Read this Instructions for Use carefully before using HYMPAVZI Prefilled Pen and each time you get a refill prescription as there may be new information.
Your healthcare provider should show you or your caregiver how to prepare and inject a dose of HYMPAVZI the right way before you use it for the first time. Do not inject yourself or someone else until you have been shown how to inject HYMPAVZI.
Important Information You Need to Know Before Injecting HYMPAVZI
- •
- Each HYMPAVZI Prefilled Pen is a Single-Dose Prefilled Pen (called “Pen” in this Instructions for Use). The HYMPAVZI Prefilled Pen contains 150 mg of HYMPAVZI for injection under the skin (subcutaneously).
- •
- Do not inject HYMPAVZI into a vein.
- •
- To help you remember when to inject HYMPAVZI, you can mark your calendar ahead of time. Call your healthcare provider if you or your caregiver have any questions about the right way to inject HYMPAVZI, or call the helpline at 1‑888‑496‑7289 (1‑888 HYMPAV‑Z).
- •
- Use HYMPAVZI exactly as prescribed by your healthcare provider.
- •
- HYMPAVZI is not made with natural rubber latex.
Storing HYMPAVZI
- •
- Store HYMPAVZI in a refrigerator at 36°F to 46°F (2°C to 8°C).
- •
- Store HYMPAVZI in the original carton to protect from light.
- •
- If needed, HYMPAVZI may be stored one time at room temperature, up to 86°F (30°C) in the original carton for up to 7 days. Do not return HYMPAVZI to the refrigerator after storing at room temperature.
- •
- Throw away (dispose of) HYMPAVZI that has been left out of the refrigerator for more than 7 days.
- •
- Do not freeze HYMPAVZI.
- •
- Do not shake HYMPAVZI.
- •
- Do not use past the expiration date (EXP) printed on the HYMPAVZI Prefilled Pen.
- •
- Keep HYMPAVZI and all medicines out of the reach of children.
Supplies Needed For HYMPAVZI Injection
Gather the following supplies on a clean flat surface:
Included in the carton:
- •
- 1 HYMPAVZI Prefilled Pen
Not included in the carton:
- •
- 1 alcohol swab
- •
- 1 cotton ball or gauze pad
- •
- 1 FDA-cleared sharps disposal container for Pen disposal (see “Step 10 – Disposal of Pen” and “Safe Pen Disposal” information section)
HYMPAVZI Prefilled Pen
Preparing to Inject HYMPAVZI
Step 1 – Getting Ready
- •
- Remove the Pen from its carton and keep out of direct sunlight.
- •
- Make sure the name HYMPAVZI appears on the carton and Pen label.
- •
- Check the Pen for any visible damage such as cracks or leaks.
- •
- Wash and dry your hands.
- •
- Do not remove the cap until you are ready to inject.
- •
- Throw away (dispose of) the Pen if it is damaged, or if the Pen or the carton containing the Pen has been dropped.
- •
- Do not use the Pen if:
- o
- it has been stored in direct light. Exposure to room light during dose preparation and injection is acceptable.
- o
- it has been frozen or thawed or it has been out of the refrigerator for more than 7 days.
Note: For a more comfortable injection, allow the Pen to warm up to room temperature in the carton away from direct sunlight for about 15 to 30 minutes.
Do not use any other methods to warm up the Pen, such as warming the Pen in a microwave or hot water.
Step 2 – Check Expiration Date
- •
- Check the expiration date (EXP) printed on the Pen label.
- •
- Do not use if the expiration date (EXP) has passed.
Step 3 – Check Medicine
- •
- Look carefully at the medicine through the window on the Pen.
- o
- The medicine should be clear and colorless to light yellow.
- o
- Do not use the Pen if the medicine is cloudy, dark yellow, or contains flakes or particles.
Note: It is normal to see air bubbles in the window.
If you have any questions about the medicine, contact your healthcare provider.
Step 4 – Choose and Clean Your Injection Site
- •
- Choose an injection site on your stomach area (abdomen) or thigh unless a different site has been suggested by your healthcare provider. HYMPAVZI may also be injected into your buttocks by a healthcare provider or caregiver only. Keep at least 2 inches away from your belly button.
- •
- Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI and away from any other medicine given under your skin. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Clean the injection site with soap and water, or an alcohol swab.
- •
- Allow the site to air dry. Do not touch, fan, or blow on the cleaned injection site.
- •
- Do not inject HYMPAVZI into bony areas or areas on your skin that are bruised, red, sore (tender) or hard. Avoid injecting into areas with scars or stretch marks.
- •
- Do not inject HYMPAVZI into a vein.
- •
- Do not inject HYMPAVZI through your clothes.
Step 5 – Twist Off Cap
- •
- Twist and pull off the cap.
- •
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
Note:
- o
- It is normal to see a few drops of medicine at the needle tip.
- o
- The needle cover will stay inside the cap after cap removal.
Caution: Handle the Pen with care as it contains a needle.
Do not put or press your hand over the needle guard. Doing so may cause a needle injury.
Injecting HYMPAVZI
Step 6 – Inject Medicine
- •
- Hold the Pen straight (at 90° angle) against your cleaned skin so you can see the window.
- •
- Push the Pen down firmly straight against your skin and keep pushing until the injection is complete. You will hear the 1st click when the injection starts.
- •
- Keep pushing the Pen firmly against your skin while the yellow bar moves across the window. You will hear a 2nd click when the injection is almost complete.
- •
- Count slowly to 5 after you hear the 2nd click to make sure you get a full dose.
Do not remove the Pen from your skin until you have counted slowly to 5 after you hear the 2nd click and until the yellow marker completely fills the window (see “Step 7 – Remove Pen”).
Note: The needle goes into your skin as you push the Pen down. Your healthcare provider may suggest gently pinching your skin while you inject.
Note: If you do not hear a click when pushing the Pen against your skin, try pushing down harder. If you still cannot start the injection, get a new HYMPAVZI Prefilled Pen.
Caution: If you change your mind where to inject after inserting the needle into your skin, you will need to throw away (dispose of) the Pen and get a new HYMPAVZI Prefilled Pen.
- •
- Remove the Pen from your skin.
- o
- If you see a small drop of medicine on your skin, wait a little longer before removing the Pen when you give your next injection.
Note: After you remove the Pen from your skin, the needle guard will automatically cover the needle and lock in place.
The Pen cannot be reused.
Step 8 – Check Window
- •
- Check the window to make sure all the medicine has been injected.
If the yellow bar is not in the position shown, this means you have not received a full dose. Call your healthcare provider for help.
Do not inject another dose.
Step 9 – After Care
- •
- Press lightly on the injection site for a few seconds with a clean cotton ball or gauze pad if you see a drop of blood.
- •
- Do not rub the area.
Note: If bleeding does not stop, contact your healthcare provider.
Note: If your prescribed dose requires 2 injections of HYMPAVZI, repeat steps 1-9. Change (rotate) the injection site each time you give yourself an injection of HYMPAVZI. You may use the same area of your body but be sure to choose a different injection site in that area.
- •
- Put the used Pen in an FDA-cleared sharps disposal container right away after use.
- •
- Do not throw away (dispose of) Pens in the household trash.
Note: If you do not have an FDA-cleared sharps disposal container, please see the “Safe Pen Disposal” information section.
- •
- Always throw away (dispose of) Pens in a sharps disposal container. Do not dispose of Pens in the household trash.
- •
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that:
- o
- is made of heavy-duty plastic,
- o
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- o
- is upright and stable during use,
- o
- is leak-resistant, and
- o
- is properly labeled to warn of hazardous waste inside the container.
- •
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to throw away (dispose of) your sharps disposal container. There may be state or local laws about how you should throw away used needles and Pens. For more information about safe sharps disposal, and for specific information about safe sharps disposal for the state you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- •
- Do not recycle your used sharps disposal container.

US License No. 2001
Distributed by
Pfizer Labs
Division of Pfizer Inc.
New York, NY 10001
LAB-1577-1.0
For more information, go to www.pfizer.com or call 1-800-438-1985.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 10/2024
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Contact Medical Information.9AM-5PM ET Monday to Friday; excluding holidays.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.