PENBRAYA™ Dosage and Administration
(meningococcal groups A, B, C, W, and Y vaccine)
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.2 Preparation
PENBRAYA is supplied in a kit that includes a vial of Lyophilized MenACWY Component (a sterile white powder), a prefilled syringe containing the MenB Component and a vial adapter.
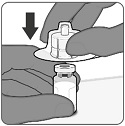
To form PENBRAYA, reconstitute the Lyophilized MenACWY Component with the MenB Component as described in the instructions below.
2.3 Administration
For intramuscular use only.
After reconstitution, PENBRAYA is a homogeneous white suspension. If the vaccine is not a homogenous suspension, shake to resuspend prior to administration. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer PENBRAYA immediately or store between 2°C and 30°C (36°F and 86°F) and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
Find PENBRAYA™ medical information:

Find PENBRAYA™ medical information:
PENBRAYA™ Quick Finder
Health Professional Information
Dosage and Administration
2 DOSAGE AND ADMINISTRATION
For intramuscular use only.
2.2 Preparation
PENBRAYA is supplied in a kit that includes a vial of Lyophilized MenACWY Component (a sterile white powder), a prefilled syringe containing the MenB Component and a vial adapter.
To form PENBRAYA, reconstitute the Lyophilized MenACWY Component with the MenB Component as described in the instructions below.
2.3 Administration
For intramuscular use only.
After reconstitution, PENBRAYA is a homogeneous white suspension. If the vaccine is not a homogenous suspension, shake to resuspend prior to administration. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer PENBRAYA immediately or store between 2°C and 30°C (36°F and 86°F) and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.