VIRACEPT Description
(nelfinavir mesylate)
11 DESCRIPTION
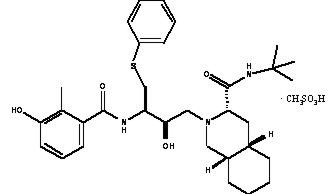
VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus type 1 (HIV-1) protease. VIRACEPT Tablets are available for oral administration as a light-blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfinavir-free base) and as a white oval tablet with a clear film coating in 625 mg strength (as nelfinavir-free base). Each tablet contains the following common inactive ingredients: calcium silicate, crospovidone, magnesium stearate, hypromellose, and triacetin. In addition, the 250 mg tablet contains FD&C blue #2 powder and the 625 mg tablet contains colloidal silicon dioxide. VIRACEPT Oral Powder is available for oral administration in a 50 mg/g strength (as nelfinavir-free base) in bottles. The oral powder also contains the following inactive ingredients: microcrystalline cellulose, maltodextrin, dibasic potassium phosphate, crospovidone, hypromellose, aspartame, sucrose palmitate, and natural and artificial flavor. The chemical name for nelfinavir mesylate is [3S-[2(2S*, 3S*), 3α,4aβ,8aβ]]-N-(1,1-dimethylethyl)decahydro-2-[2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-3-isoquinoline carboxamide mono-methanesulfonate (salt) and the molecular weight is 663.90 (567.79 as the free base). Nelfinavir mesylate has the following structural formula:
Nelfinavir mesylate is a white to off-white amorphous powder, slightly soluble in water at pH ≤4 and freely soluble in methanol, ethanol, 2-propanol and propylene glycol.
Find VIRACEPT medical information:

Find VIRACEPT medical information:
VIRACEPT Quick Finder
Health Professional Information
Description
11 DESCRIPTION
VIRACEPT (nelfinavir mesylate) is an inhibitor of the human immunodeficiency virus type 1 (HIV-1) protease. VIRACEPT Tablets are available for oral administration as a light-blue, capsule-shaped tablet with a clear film coating in 250 mg strength (as nelfinavir-free base) and as a white oval tablet with a clear film coating in 625 mg strength (as nelfinavir-free base). Each tablet contains the following common inactive ingredients: calcium silicate, crospovidone, magnesium stearate, hypromellose, and triacetin. In addition, the 250 mg tablet contains FD&C blue #2 powder and the 625 mg tablet contains colloidal silicon dioxide. VIRACEPT Oral Powder is available for oral administration in a 50 mg/g strength (as nelfinavir-free base) in bottles. The oral powder also contains the following inactive ingredients: microcrystalline cellulose, maltodextrin, dibasic potassium phosphate, crospovidone, hypromellose, aspartame, sucrose palmitate, and natural and artificial flavor. The chemical name for nelfinavir mesylate is [3S-[2(2S*, 3S*), 3α,4aβ,8aβ]]-N-(1,1-dimethylethyl)decahydro-2-[2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-(phenylthio)butyl]-3-isoquinoline carboxamide mono-methanesulfonate (salt) and the molecular weight is 663.90 (567.79 as the free base). Nelfinavir mesylate has the following structural formula:
Nelfinavir mesylate is a white to off-white amorphous powder, slightly soluble in water at pH ≤4 and freely soluble in methanol, ethanol, 2-propanol and propylene glycol.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.