XALKORI® Clinical Studies
(crizotinib)
14 CLINICAL STUDIES
14.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
Previously Untreated ALK-Positive Metastatic NSCLC - Study 1 (PROFILE 1014; NCT01154140)
The efficacy of XALKORI for the treatment of patients with ALK-positive metastatic NSCLC, who had not received previous systemic treatment for advanced disease, was demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 1). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart fluorescence in situ hybridization (FISH) Probe Kit, prior to randomization. The major efficacy outcome measure was progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as assessed by independent radiology review (IRR) committee. Additional efficacy outcome measures included objective response rate (ORR) as assessed by IRR, DOR, and overall survival (OS). Patient-reported lung cancer symptoms were assessed at baseline and periodically during treatment.
Patients were randomized to receive XALKORI (n=172) or chemotherapy (n=171). Randomization was stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0–1, 2), race (Asian, non-Asian), and brain metastases (present, absent). Patients in the XALKORI arm received XALKORI 250 mg orally twice daily until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Chemotherapy consisted of pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 or carboplatin AUC of 5 or 6 mg×min/mL by intravenous infusion every 3 weeks for up to 6 cycles. Patients in the chemotherapy arm were not permitted to receive maintenance chemotherapy. At the time of documented disease progression, as per independent radiology review, patients randomized to chemotherapy were offered XALKORI.
The demographic characteristics of the overall study population were 62% female, median age of 53 years, baseline ECOG performance status 0 or 1 (95%), 51% White and 46% Asian, 4% current smokers, 32% past smokers, and 64% never smokers. The disease characteristics of the overall study population were metastatic disease in 98% of patients, 92% of patients' tumors were classified as adenocarcinoma histology, 27% of patients had brain metastases, and 7% received systemic chemotherapy as adjuvant or neoadjuvant therapy. At the time of the final analysis of overall survival, 84% of patients randomized to the chemotherapy arm subsequently received XALKORI.
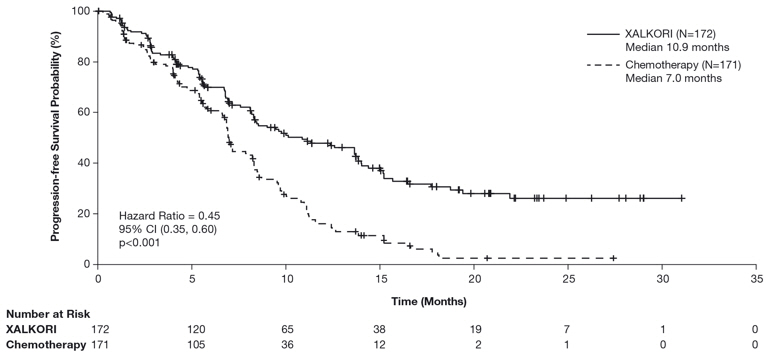
Study 1 demonstrated a statistically significant improvement in PFS in patients treated with XALKORI. There was no statistically significant difference in OS between patients treated with XALKORI and patients treated with chemotherapy. Table 16 and Figure 1 summarize the efficacy results. Exploratory patient-reported symptom measures of baseline and post-treatment dyspnea, cough, and chest pain suggested a delay in time to development of or worsening of dyspnea, but not cough or chest pain, in patients treated with XALKORI as compared to chemotherapy. The patient-reported delay in onset or worsening of dyspnea may be an overestimation because patients were not blinded to treatment assignment.
| XALKORI (N=172) | Chemotherapy (N=171) | |
|---|---|---|
| HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; NR=not reached; CR=complete response; PR=partial response. | ||
Progression-Free Survival (Based on IRR) | ||
Number of Events (%) | 100 (58%) | 137 (80%) |
Progressive Disease | 89 (52%) | 132 (77%) |
Death | 11 (6%) | 5 (3%) |
Median, Months (95% CI) | 10.9 (8.3, 13.9) | 7.0 (6.8, 8.2) |
HR (95% CI)* | 0.45 (0.35, 0.60) | |
p-value† | <0.001 | |
Overall Survival | ||
Number of Events (%) | 71 (41%) | 81 (47%) |
Median, Months (95% CI) | NR (45.8, NR) | 47.5 (32.2, NR) |
HR (95% CI)* | 0.76 (0.55, 1.05) | |
p-value† | 0.098 | |
Tumor Responses (Based on IRR) | ||
Objective Response Rate % (95% CI) | 74% (67, 81) | 45% (37, 53) |
CR, n (%) | 3 (1.7%) | 2 (1.2%) |
PR, n (%) | 125 (73%) | 75 (44%) |
p-value‡ | <0.001 | |
Duration of Response | ||
Median, Months (95% CI) | 11.3 (8.1, 13.8) | 5.3 (4.1, 5.8) |
Figure 1. Kaplan-Meier Curves of Progression-Free Survival as Assessed by IRR in Study 1 |
 |
Previously Treated ALK-Positive Metastatic NSCLC - Study 2 (PROFILE 1007; NCT00932893)
The efficacy of XALKORI as monotherapy for the treatment of 347 patients with ALK-positive metastatic NSCLC, previously treated with 1 platinum-based chemotherapy regimen, were demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 2). The major efficacy outcome was PFS according to RECIST version 1.1 as assessed by IRR. Additional efficacy outcomes included ORR as assessed by IRR, DOR, and OS.
Patients were randomized to receive XALKORI 250 mg orally twice daily (n=173) or chemotherapy (n=174). Chemotherapy consisted of pemetrexed 500 mg/m2 (if pemetrexed-naïve; n=99) or docetaxel 75 mg/m2 (n=72) intravenously (IV) every 21 days. Patients in both treatment arms continued treatment until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Randomization was stratified by ECOG performance status (0–1, 2), brain metastases (present, absent), and prior EGFR tyrosine kinase inhibitor treatment (yes, no). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart FISH Probe Kit, prior to randomization.
The demographic characteristics of the overall study population were 56% female, median age of 50 years, baseline ECOG performance status 0 or 1 (90%), 52% White and 45% Asian, 4% current smokers, 33% past smokers, and 63% never smokers. The disease characteristics of the overall study population were metastatic disease in at least 95% of patients and at least 93% of patients' tumors were classified as adenocarcinoma histology. At the time of the final analysis of overall survival, 89% of patients randomized to the chemotherapy arm subsequently received XALKORI.
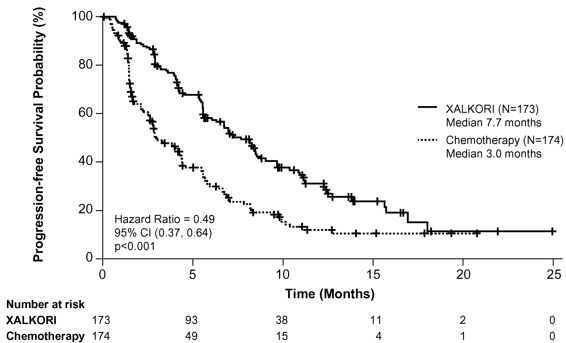
Study 2 demonstrated a statistically significant improvement in PFS in the patients treated with XALKORI. Table 17 and Figure 2 summarize the efficacy results.
| XALKORI (N=173) | Chemotherapy (N=174) | |
|---|---|---|
| HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; CR=complete response; PR=partial response. | ||
Progression-Free Survival (Based on IRR) | ||
Number of Events (%) | 100 (58%) | 127 (73%) |
Progressive Disease | 84 (49%) | 119 (68%) |
Death | 16 (9%) | 8 (5%) |
Median, Months (95% CI) | 7.7 (6.0, 8.8) | 3.0* (2.6, 4.3) |
HR (95% CI)† | 0.49 (0.37, 0.64) | |
p-value‡ | <0.001 | |
Overall Survival | ||
Number of Events (%) | 116 (67%) | 126 (72%) |
Median, Months (95% CI) | 21.7 (18.9,30.5) | 21.9 (16.8,26.0) |
HR (95% CI)† | 0.85 (0.66, 1.10) | |
p-value‡ | 0.229 | |
Tumor Responses (Based on IRR) | ||
Objective Response Rate % (95% CI) | 65% (58, 72) | 20% (14, 26) |
CR, n (%) | 1 (0.6%) | 0 |
PR, n (%) | 112 (65%) | 34 (20%) |
p-value§ | <0.001 | |
Duration of Response | ||
Median, Months (95% CI) | 7.4 (6.1, 9.7) | 5.6 (3.4, 8.3) |
Figure 2. Kaplan-Meier Curves of Progression-Free Survival as Assessed by IRR in Study 2 |
 |
ROS1-Positive Metastatic NSCLC - Study 3 (PROFILE 1001; NCT00585195)
The efficacy and safety of XALKORI was investigated in a multicenter, single-arm study (Study 3), in which patients with ROS1-positive metastatic NSCLC received XALKORI 250 mg orally twice daily. Patients were required to have histologically-confirmed advanced NSCLC with a ROS1 rearrangement, age 18 years or older, ECOG performance status of 0, 1, or 2, and measurable disease. The efficacy outcome measures were ORR and DOR according to RECIST version 1.0 as assessed by IRR and investigator, with imaging performed every 8 weeks for the first 60 weeks.
Baseline demographic and disease characteristics were female (56%), median age of 53 years, baseline ECOG performance status of 0 or 1 (98%), White (54%), Asian (42%), past smokers (22%), never smokers (78%), metastatic disease (92%), adenocarcinoma (96%), no prior systemic therapy for metastatic disease (14%), and prior platinum-based chemotherapy for metastatic disease (80%). The ROS1 status of NSCLC tissue samples was determined by laboratory-developed break-apart FISH (96%) or RT-PCR (4%) clinical trial assays. For assessment by FISH, ROS1 positivity required that ≥15% of a minimum of 50 evaluated nuclei contained a ROS1 gene rearrangement.
Efficacy results are summarized in Table 18.
| Efficacy Parameters | IRR (N=50) | Investigator-Assessed (N=50) |
|---|---|---|
| IRR=independent radiology review; CI=confidence interval; NR=not reached. | ||
| ||
Objective Response Rate (95% CI) | 66% (51, 79) | 72% (58, 84) |
Complete Response, n | 1 | 5 |
Partial Response, n | 32 | 31 |
Duration of Response | ||
Median, Months (95% CI) | 18.3 (12.7, NR) | NR (14.5, NR) |
14.2 Relapsed or Refractory, Systemic ALK-Positive Anaplastic Large Cell Lymphoma
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single arm, open-label study in patients 1 to ≤21 years of age that included 26 patients with relapsed or refractory, systemic ALK-positive ALCL after at least one systemic treatment. ALK-positive status (confirmation of an ALK fusion) was determined locally by immunohistochemistry or fluorescence in situ hybridization. The study excluded patients with primary cutaneous ALCL or central nervous system involvement by lymphoma.
Patients received XALKORI 280 mg/m2 (20 patients) or 165 mg/m2 (6 patients) orally twice daily until disease progression or unacceptable toxicity. Patients were permitted to discontinue XALKORI to undergo hematopoietic stem cell transplantation (HSCT).
Of the 26 patients evaluated, the median age was 11 years (range: 3 to 20); 69% were male, 54% were White, 19% Black, 8% Asian. Patient enrollment by age category was 4 patients from 3 to <6 years, 11 patients from 6 to <12 years, 7 patients from 12 to <18 years, and 4 patients from 18 to ≤21 years.
All patients had received multi-agent systemic therapy, 2 (8%) had received a prior HSCT and 4 (15%) had received at least 3 prior therapies.
Efficacy was based on objective response rate and duration of response, as assessed by an independent review committee (Table 19). The median time to first response was 3.9 weeks (range: 3.5 to 9.1 weeks).
| Efficacy Parameter | N=26 |
|---|---|
| CI=confidence interval; N/n=number of patients | |
88% (71, 96) | |
Complete response, n | 21 (81%) |
Partial response, n | 2 (8%) |
Duration of response‡ | |
Patients maintaining response at 3 months, n/N | 13/23 (57%) |
Patients maintaining response at 6 months, n/N | 9/23 (39%) |
Patients maintaining response at 12 months, n/N | 5/23 (22%) |
14.3 Unresectable, Recurrent, or Refractory ALK-Positive Inflammatory Myofibroblastic Tumor
Pediatric Patients with ALK-positive IMT
Study ADVL0912
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single-arm, open-label study in patients 1 to ≤21 years of age that included 14 pediatric patients with unresectable, recurrent, or refractory ALK-positive IMT. Patients were required to have an ALK fusion determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients (n=12) received XALKORI 280 mg/m2 twice daily until disease progression or unacceptable toxicity. Two patients received a lower dose.
The demographic characteristics were median age 6.5 years (range: 2 to 13); 64% female; 71% White; 7% Black, 21% unknown; 21% Hispanic; and 71% had a Lansky/Karnofsky Score of 100. Patient enrollment by age was 4 patients from 2 to <6 years, 8 patients from 6 to <12 years, and 2 patients from 12 to <18 years.
A total of 12 (86%) patients received prior therapy. The most common prior therapy was surgery (57%).
The major efficacy outcome was objective response rate according to RECIST version 1.0 as assessed by an independent review committee (Table 20).
| Efficacy Parameter | N=14 |
|---|---|
| CI=confidence interval; N/n=number of patients. | |
Objective response rate (95% CI, %)* | 86% (57, 98) |
Complete response, n (%) | 5 (36) |
Partial response n (%) | 7 (50) |
Duration of response† | N=12 |
≥6 months, n (%) | 7 (58) |
≥12 months, n (%) | 7 (58) |
Adult Patients with ALK-positive IMT
Study A8081013
The efficacy of XALKORI was evaluated in Study A8081013 (NCT01121588), a multicenter, single-arm, open-label study that included 7 adult patients with unresectable, recurrent, or refractory ALK-positive IMT. ALK fusion was determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients received XALKORI 250 mg twice daily.
The demographic characteristics were median age 38 years (range: 23 to 73); 57% male; 57% White, 43% Asian; and 86% ECOG performance status of 0 or 1. Two (29%) patients had at least one prior systemic treatment.
The major efficacy outcome was objective response rate according to RECIST version 1.1 per investigator assessment. For the 7 patients with ALK-positive IMT, 5 experienced a response including 1 complete response. The DOR was ≥6 months for all 5 patients and ≥12 months for 2 patients.
Find XALKORI® medical information:

Find XALKORI® medical information:
XALKORI® Quick Finder
Health Professional Information
Clinical Studies
14 CLINICAL STUDIES
14.1 ALK- or ROS1-Positive Metastatic Non-Small Cell Lung Cancer
Previously Untreated ALK-Positive Metastatic NSCLC - Study 1 (PROFILE 1014; NCT01154140)
The efficacy of XALKORI for the treatment of patients with ALK-positive metastatic NSCLC, who had not received previous systemic treatment for advanced disease, was demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 1). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart fluorescence in situ hybridization (FISH) Probe Kit, prior to randomization. The major efficacy outcome measure was progression-free survival (PFS) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as assessed by independent radiology review (IRR) committee. Additional efficacy outcome measures included objective response rate (ORR) as assessed by IRR, DOR, and overall survival (OS). Patient-reported lung cancer symptoms were assessed at baseline and periodically during treatment.
Patients were randomized to receive XALKORI (n=172) or chemotherapy (n=171). Randomization was stratified by Eastern Cooperative Oncology Group (ECOG) performance status (0–1, 2), race (Asian, non-Asian), and brain metastases (present, absent). Patients in the XALKORI arm received XALKORI 250 mg orally twice daily until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Chemotherapy consisted of pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 or carboplatin AUC of 5 or 6 mg×min/mL by intravenous infusion every 3 weeks for up to 6 cycles. Patients in the chemotherapy arm were not permitted to receive maintenance chemotherapy. At the time of documented disease progression, as per independent radiology review, patients randomized to chemotherapy were offered XALKORI.
The demographic characteristics of the overall study population were 62% female, median age of 53 years, baseline ECOG performance status 0 or 1 (95%), 51% White and 46% Asian, 4% current smokers, 32% past smokers, and 64% never smokers. The disease characteristics of the overall study population were metastatic disease in 98% of patients, 92% of patients' tumors were classified as adenocarcinoma histology, 27% of patients had brain metastases, and 7% received systemic chemotherapy as adjuvant or neoadjuvant therapy. At the time of the final analysis of overall survival, 84% of patients randomized to the chemotherapy arm subsequently received XALKORI.
Study 1 demonstrated a statistically significant improvement in PFS in patients treated with XALKORI. There was no statistically significant difference in OS between patients treated with XALKORI and patients treated with chemotherapy. Table 16 and Figure 1 summarize the efficacy results. Exploratory patient-reported symptom measures of baseline and post-treatment dyspnea, cough, and chest pain suggested a delay in time to development of or worsening of dyspnea, but not cough or chest pain, in patients treated with XALKORI as compared to chemotherapy. The patient-reported delay in onset or worsening of dyspnea may be an overestimation because patients were not blinded to treatment assignment.
| XALKORI (N=172) | Chemotherapy (N=171) | |
|---|---|---|
| HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; NR=not reached; CR=complete response; PR=partial response. | ||
Progression-Free Survival (Based on IRR) | ||
Number of Events (%) | 100 (58%) | 137 (80%) |
Progressive Disease | 89 (52%) | 132 (77%) |
Death | 11 (6%) | 5 (3%) |
Median, Months (95% CI) | 10.9 (8.3, 13.9) | 7.0 (6.8, 8.2) |
HR (95% CI)* | 0.45 (0.35, 0.60) | |
p-value† | <0.001 | |
Overall Survival | ||
Number of Events (%) | 71 (41%) | 81 (47%) |
Median, Months (95% CI) | NR (45.8, NR) | 47.5 (32.2, NR) |
HR (95% CI)* | 0.76 (0.55, 1.05) | |
p-value† | 0.098 | |
Tumor Responses (Based on IRR) | ||
Objective Response Rate % (95% CI) | 74% (67, 81) | 45% (37, 53) |
CR, n (%) | 3 (1.7%) | 2 (1.2%) |
PR, n (%) | 125 (73%) | 75 (44%) |
p-value‡ | <0.001 | |
Duration of Response | ||
Median, Months (95% CI) | 11.3 (8.1, 13.8) | 5.3 (4.1, 5.8) |
Previously Treated ALK-Positive Metastatic NSCLC - Study 2 (PROFILE 1007; NCT00932893)
The efficacy of XALKORI as monotherapy for the treatment of 347 patients with ALK-positive metastatic NSCLC, previously treated with 1 platinum-based chemotherapy regimen, were demonstrated in a randomized, multicenter, open-label, active-controlled study (Study 2). The major efficacy outcome was PFS according to RECIST version 1.1 as assessed by IRR. Additional efficacy outcomes included ORR as assessed by IRR, DOR, and OS.
Patients were randomized to receive XALKORI 250 mg orally twice daily (n=173) or chemotherapy (n=174). Chemotherapy consisted of pemetrexed 500 mg/m2 (if pemetrexed-naïve; n=99) or docetaxel 75 mg/m2 (n=72) intravenously (IV) every 21 days. Patients in both treatment arms continued treatment until documented disease progression, intolerance to therapy, or the investigator determined that the patient was no longer experiencing clinical benefit. Randomization was stratified by ECOG performance status (0–1, 2), brain metastases (present, absent), and prior EGFR tyrosine kinase inhibitor treatment (yes, no). Patients were required to have ALK-positive NSCLC as identified by the FDA-approved assay, Vysis ALK Break-Apart FISH Probe Kit, prior to randomization.
The demographic characteristics of the overall study population were 56% female, median age of 50 years, baseline ECOG performance status 0 or 1 (90%), 52% White and 45% Asian, 4% current smokers, 33% past smokers, and 63% never smokers. The disease characteristics of the overall study population were metastatic disease in at least 95% of patients and at least 93% of patients' tumors were classified as adenocarcinoma histology. At the time of the final analysis of overall survival, 89% of patients randomized to the chemotherapy arm subsequently received XALKORI.
Study 2 demonstrated a statistically significant improvement in PFS in the patients treated with XALKORI. Table 17 and Figure 2 summarize the efficacy results.
| XALKORI (N=173) | Chemotherapy (N=174) | |
|---|---|---|
| HR=hazard ratio; CI=confidence interval; IRR=independent radiology review; CR=complete response; PR=partial response. | ||
Progression-Free Survival (Based on IRR) | ||
Number of Events (%) | 100 (58%) | 127 (73%) |
Progressive Disease | 84 (49%) | 119 (68%) |
Death | 16 (9%) | 8 (5%) |
Median, Months (95% CI) | 7.7 (6.0, 8.8) | 3.0* (2.6, 4.3) |
HR (95% CI)† | 0.49 (0.37, 0.64) | |
p-value‡ | <0.001 | |
Overall Survival | ||
Number of Events (%) | 116 (67%) | 126 (72%) |
Median, Months (95% CI) | 21.7 (18.9,30.5) | 21.9 (16.8,26.0) |
HR (95% CI)† | 0.85 (0.66, 1.10) | |
p-value‡ | 0.229 | |
Tumor Responses (Based on IRR) | ||
Objective Response Rate % (95% CI) | 65% (58, 72) | 20% (14, 26) |
CR, n (%) | 1 (0.6%) | 0 |
PR, n (%) | 112 (65%) | 34 (20%) |
p-value§ | <0.001 | |
Duration of Response | ||
Median, Months (95% CI) | 7.4 (6.1, 9.7) | 5.6 (3.4, 8.3) |
ROS1-Positive Metastatic NSCLC - Study 3 (PROFILE 1001; NCT00585195)
The efficacy and safety of XALKORI was investigated in a multicenter, single-arm study (Study 3), in which patients with ROS1-positive metastatic NSCLC received XALKORI 250 mg orally twice daily. Patients were required to have histologically-confirmed advanced NSCLC with a ROS1 rearrangement, age 18 years or older, ECOG performance status of 0, 1, or 2, and measurable disease. The efficacy outcome measures were ORR and DOR according to RECIST version 1.0 as assessed by IRR and investigator, with imaging performed every 8 weeks for the first 60 weeks.
Baseline demographic and disease characteristics were female (56%), median age of 53 years, baseline ECOG performance status of 0 or 1 (98%), White (54%), Asian (42%), past smokers (22%), never smokers (78%), metastatic disease (92%), adenocarcinoma (96%), no prior systemic therapy for metastatic disease (14%), and prior platinum-based chemotherapy for metastatic disease (80%). The ROS1 status of NSCLC tissue samples was determined by laboratory-developed break-apart FISH (96%) or RT-PCR (4%) clinical trial assays. For assessment by FISH, ROS1 positivity required that ≥15% of a minimum of 50 evaluated nuclei contained a ROS1 gene rearrangement.
Efficacy results are summarized in Table 18.
| Efficacy Parameters | IRR (N=50) | Investigator-Assessed (N=50) |
|---|---|---|
| IRR=independent radiology review; CI=confidence interval; NR=not reached. | ||
| ||
Objective Response Rate (95% CI) | 66% (51, 79) | 72% (58, 84) |
Complete Response, n | 1 | 5 |
Partial Response, n | 32 | 31 |
Duration of Response | ||
Median, Months (95% CI) | 18.3 (12.7, NR) | NR (14.5, NR) |
14.2 Relapsed or Refractory, Systemic ALK-Positive Anaplastic Large Cell Lymphoma
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single arm, open-label study in patients 1 to ≤21 years of age that included 26 patients with relapsed or refractory, systemic ALK-positive ALCL after at least one systemic treatment. ALK-positive status (confirmation of an ALK fusion) was determined locally by immunohistochemistry or fluorescence in situ hybridization. The study excluded patients with primary cutaneous ALCL or central nervous system involvement by lymphoma.
Patients received XALKORI 280 mg/m2 (20 patients) or 165 mg/m2 (6 patients) orally twice daily until disease progression or unacceptable toxicity. Patients were permitted to discontinue XALKORI to undergo hematopoietic stem cell transplantation (HSCT).
Of the 26 patients evaluated, the median age was 11 years (range: 3 to 20); 69% were male, 54% were White, 19% Black, 8% Asian. Patient enrollment by age category was 4 patients from 3 to <6 years, 11 patients from 6 to <12 years, 7 patients from 12 to <18 years, and 4 patients from 18 to ≤21 years.
All patients had received multi-agent systemic therapy, 2 (8%) had received a prior HSCT and 4 (15%) had received at least 3 prior therapies.
Efficacy was based on objective response rate and duration of response, as assessed by an independent review committee (Table 19). The median time to first response was 3.9 weeks (range: 3.5 to 9.1 weeks).
| Efficacy Parameter | N=26 |
|---|---|
| CI=confidence interval; N/n=number of patients | |
88% (71, 96) | |
Complete response, n | 21 (81%) |
Partial response, n | 2 (8%) |
Duration of response‡ | |
Patients maintaining response at 3 months, n/N | 13/23 (57%) |
Patients maintaining response at 6 months, n/N | 9/23 (39%) |
Patients maintaining response at 12 months, n/N | 5/23 (22%) |
14.3 Unresectable, Recurrent, or Refractory ALK-Positive Inflammatory Myofibroblastic Tumor
Pediatric Patients with ALK-positive IMT
Study ADVL0912
The efficacy of XALKORI was evaluated in Study ADVL0912 (NCT00939770), a multicenter, single-arm, open-label study in patients 1 to ≤21 years of age that included 14 pediatric patients with unresectable, recurrent, or refractory ALK-positive IMT. Patients were required to have an ALK fusion determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients (n=12) received XALKORI 280 mg/m2 twice daily until disease progression or unacceptable toxicity. Two patients received a lower dose.
The demographic characteristics were median age 6.5 years (range: 2 to 13); 64% female; 71% White; 7% Black, 21% unknown; 21% Hispanic; and 71% had a Lansky/Karnofsky Score of 100. Patient enrollment by age was 4 patients from 2 to <6 years, 8 patients from 6 to <12 years, and 2 patients from 12 to <18 years.
A total of 12 (86%) patients received prior therapy. The most common prior therapy was surgery (57%).
The major efficacy outcome was objective response rate according to RECIST version 1.0 as assessed by an independent review committee (Table 20).
| Efficacy Parameter | N=14 |
|---|---|
| CI=confidence interval; N/n=number of patients. | |
Objective response rate (95% CI, %)* | 86% (57, 98) |
Complete response, n (%) | 5 (36) |
Partial response n (%) | 7 (50) |
Duration of response† | N=12 |
≥6 months, n (%) | 7 (58) |
≥12 months, n (%) | 7 (58) |
Adult Patients with ALK-positive IMT
Study A8081013
The efficacy of XALKORI was evaluated in Study A8081013 (NCT01121588), a multicenter, single-arm, open-label study that included 7 adult patients with unresectable, recurrent, or refractory ALK-positive IMT. ALK fusion was determined locally by immunohistochemistry or fluorescence in situ hybridization. Patients received XALKORI 250 mg twice daily.
The demographic characteristics were median age 38 years (range: 23 to 73); 57% male; 57% White, 43% Asian; and 86% ECOG performance status of 0 or 1. Two (29%) patients had at least one prior systemic treatment.
The major efficacy outcome was objective response rate according to RECIST version 1.1 per investigator assessment. For the 7 patients with ALK-positive IMT, 5 experienced a response including 1 complete response. The DOR was ≥6 months for all 5 patients and ≥12 months for 2 patients.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.