XALKORI® Description
(crizotinib)
11 DESCRIPTION
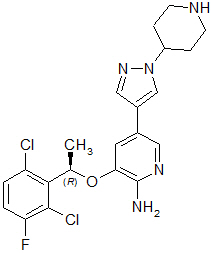
Crizotinib is a kinase inhibitor. The molecular formula for crizotinib is C21H22Cl2FN5O and the molecular weight is 450.34 daltons. Crizotinib is described chemically as (R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine.
The chemical structure of crizotinib is shown below:
Crizotinib is a white to pale-yellow powder with a pKa of 9.4 (piperidinium cation) and 5.6 (pyridinium cation). The solubility of crizotinib in aqueous media decreases over the range pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65.
Capsules:
XALKORI (crizotinib) capsules for oral administration are supplied as printed hard-shell capsules containing 250 mg or 200 mg of crizotinib together with colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, and hard gelatin capsule shells as inactive ingredients.
The pink opaque capsule shell components contain gelatin, titanium dioxide, and red iron oxide. The white opaque capsule shell components contain gelatin and titanium dioxide. The printing ink contains shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Oral Pellets:
XALKORI (crizotinib) oral pellets for oral administration are supplied as 20 mg, 50 mg, 150 mg of crizotinib contained in hard gelatin capsules. The inactive ingredients in the uncoated pellets are poloxamer and stearyl alcohol. The film-coating consists of hypromellose, glyceryl monostearate, medium chain triglycerides, polyethylene glycol/macrogol, sucrose, and talc.
Find XALKORI® medical information:
Find XALKORI® medical information:
XALKORI® Quick Finder
Health Professional Information
Description
11 DESCRIPTION
Crizotinib is a kinase inhibitor. The molecular formula for crizotinib is C21H22Cl2FN5O and the molecular weight is 450.34 daltons. Crizotinib is described chemically as (R)-3-[1-(2,6-Dichloro-3-fluorophenyl)ethoxy]-5-[1-(piperidin-4-yl)-1H-pyrazol-4-yl]pyridin-2-amine.
The chemical structure of crizotinib is shown below:
Crizotinib is a white to pale-yellow powder with a pKa of 9.4 (piperidinium cation) and 5.6 (pyridinium cation). The solubility of crizotinib in aqueous media decreases over the range pH 1.6 to pH 8.2 from greater than 10 mg/mL to less than 0.1 mg/mL. The log of the distribution coefficient (octanol/water) at pH 7.4 is 1.65.
Capsules:
XALKORI (crizotinib) capsules for oral administration are supplied as printed hard-shell capsules containing 250 mg or 200 mg of crizotinib together with colloidal silicon dioxide, microcrystalline cellulose, anhydrous dibasic calcium phosphate, sodium starch glycolate, magnesium stearate, and hard gelatin capsule shells as inactive ingredients.
The pink opaque capsule shell components contain gelatin, titanium dioxide, and red iron oxide. The white opaque capsule shell components contain gelatin and titanium dioxide. The printing ink contains shellac, propylene glycol, strong ammonia solution, potassium hydroxide, and black iron oxide.
Oral Pellets:
XALKORI (crizotinib) oral pellets for oral administration are supplied as 20 mg, 50 mg, 150 mg of crizotinib contained in hard gelatin capsules. The inactive ingredients in the uncoated pellets are poloxamer and stearyl alcohol. The film-coating consists of hypromellose, glyceryl monostearate, medium chain triglycerides, polyethylene glycol/macrogol, sucrose, and talc.
Health Professional Information
{{section_name_patient}}
{{section_body_html_patient}}
Resources
Didn’t find what you were looking for? Contact us.
Chat online with Pfizer Medical Information regarding your inquiry on a Pfizer medicine.
*Speak with a Pfizer Medical Information Professional regarding your medical inquiry. Available 9AM-5PM ET Monday to Friday; excluding holidays.
Submit a medical question for Pfizer prescription products.
Report Adverse Event
Pfizer Safety
To report an adverse event related to the Pfizer-BioNTech COVID-19 Vaccine, and you are not part of a clinical trial* for this product, click the link below to submit your information:
Pfizer Safety Reporting Site*If you are involved in a clinical trial for this product, adverse events should be reported to your coordinating study site.
If you cannot use the above website, or would like to report an adverse event related to a different Pfizer product, please call Pfizer Safety at (800) 438-1985.
FDA Medwatch
You may also contact the U.S. Food and Drug Administration (FDA) directly to report adverse events or product quality concerns either online at www.fda.gov/medwatch or call (800) 822-7967.